Polarization of an ionic bond means the distortion of the electron cloud of an anion towards a cation Polarization of an ionic bond results in an ionic. - ppt download

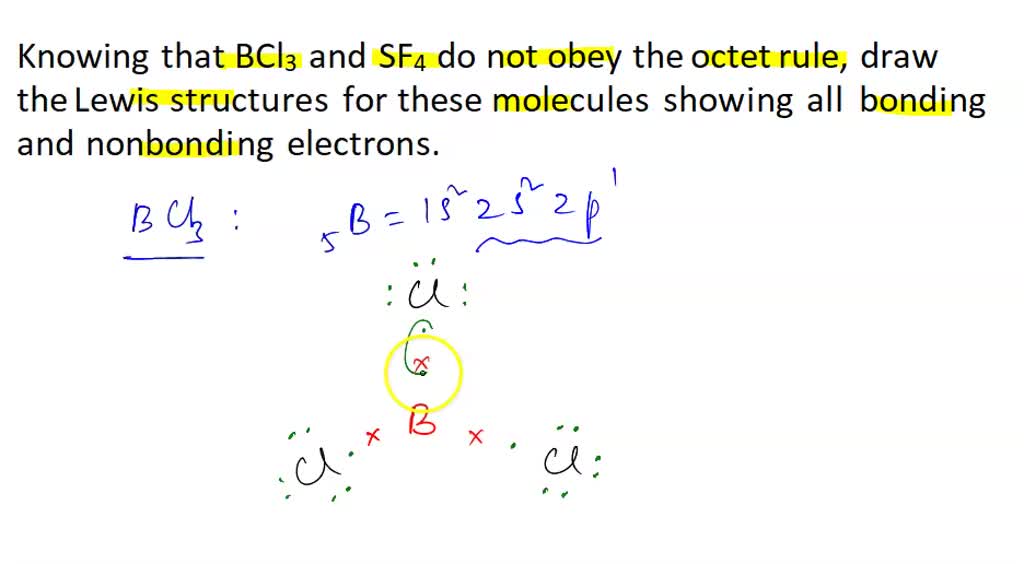
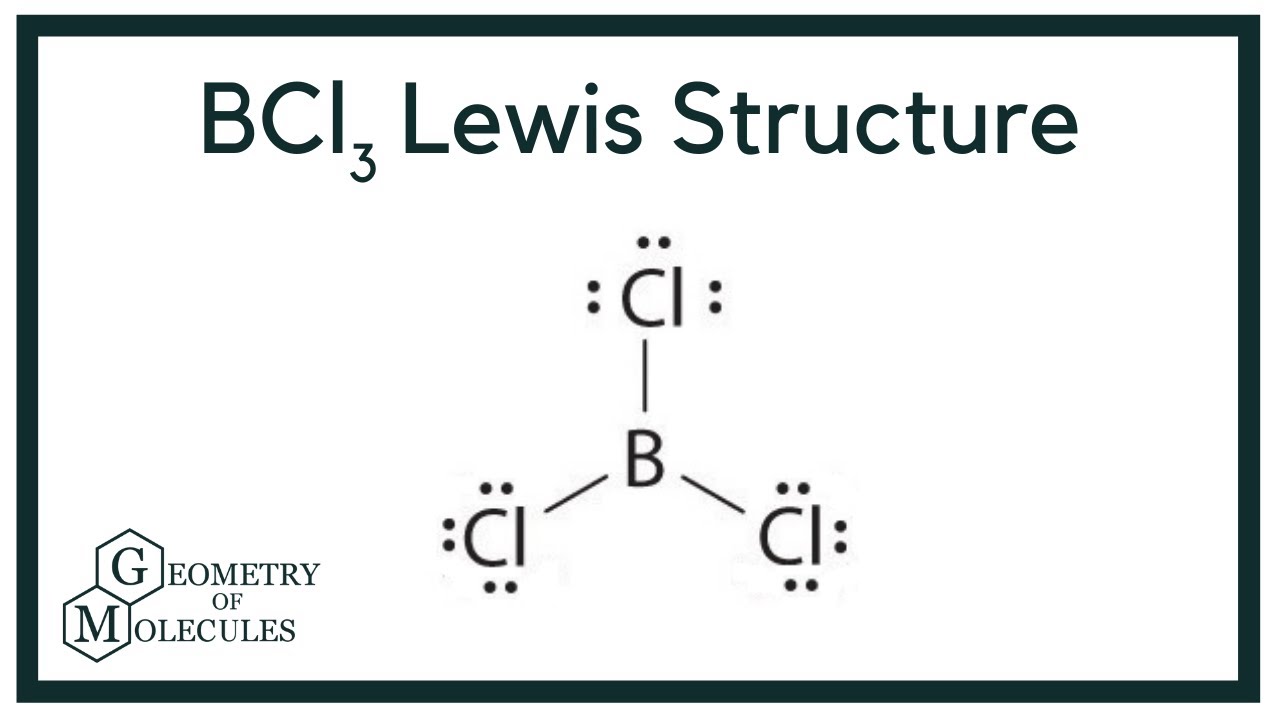
Draw the Lewis structure for BCl3. Determine its electron geometry, the number of non-bonding domains on the central atom, and the polarity of the molecule. | Homework.Study.com

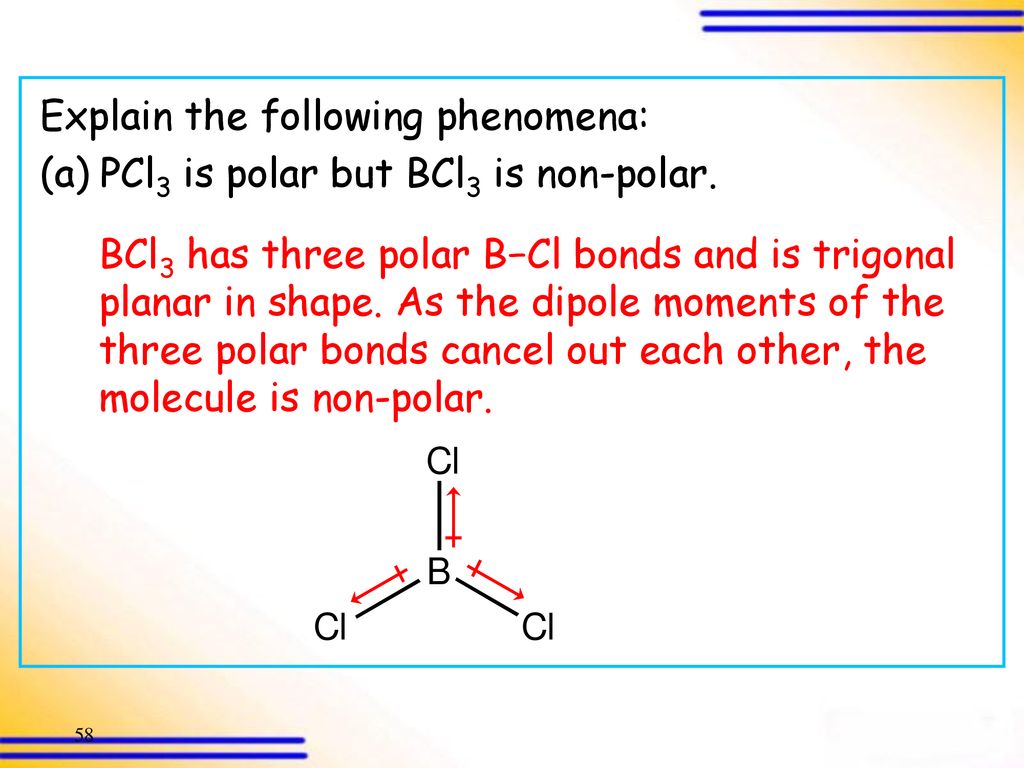
Consider the following statements about BCl3 molecules. Which statement is false? A. The B-Cl bonds are quite polar. B. The bond dipoles exactly cancel. C. The Cl-B-Cl bond angles are 109.5 degrees.

BCl3 lewis structure, molecular geometry, polar or nonpolar, hybridization, Bond angle | Molecular geometry, Molecular, Vsepr theory